 Image via Wikipedia Fritz French, Chief Executive Officer, Marcadia Biotech, Carmel, Ind.
Image via Wikipedia Fritz French, Chief Executive Officer, Marcadia Biotech, Carmel, Ind.Drug Discovery & Development - August 16, 2010
Diabetes is a common chronic disease affecting an estimated 285 million adults worldwide. This number will continue to grow as the population ages and becomes more obese—both risk factors for type 2 diabetes. The diabetes treatment market generated over $25 billion in 2009 and the number of patients is continuously rising.
Type 2 diabetes occurs when the pancreas produces insufficient amounts of insulin and/or the body’s cells become resistant to the action of insulin. Diabetes can lead to significant complications such as cardiovascular diseases, renal diseases, blindness, and amputations. Diabetic patients require ongoing treatment to maintain normal blood glucose levels.
Multiple therapeutic options are available for the treatment of diabetes, such as insulin secretagogues and insulin sensitizers, which focus on management of blood glucose and the indicator of mean blood glucose, hemoglobin A1c (HbA1c). As monotherapy fails, additional agents are added to stabilize glycemic levels. The majority of patients are treated with multiple drugs. Less than 50% of U.S. adults with type 2 diabetes reach the desired HbA1c level of less than 7%, while adverse events, such as weight gain, edema, or hypoglycemia, may undermine the therapeutic effect.
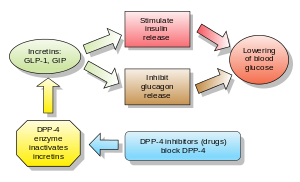
In recent years, treatment strategies have been focused on the development of novel therapeutic options, especially new drugs targeting the incretin system. Incretins are hormones secreted by the entero-endocrine cells of the gut and their impairment plays a central role in glucose homeostastis. Incretin levels are very low during fasting, but increase rapidly after eating. Orally ingested glucose triggers a significantly greater insulin secretory response than intravenous administered glucose, the so-called “incretin effect.”
The incretin-effect accounts for an estimated 50% to 70% of the total insulin secreted following glucose intake. The two relevant physiological intestinal incretin hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), account for most of the incretin effect and induce meal-stimulated insulin secretion and other related physiological processes, such as gut motility. Both incretin hormones are rapidly inactivated by the ubiquitously expressed proteolytic enzyme dipeptidyl peptidase-4 (DPP-4).
Type 2 diabetes patients have a significantly reduced incretin effect, including reduced secretion of GLP-1 and a resistance to the insulinotropic effect of GIP. Consequently, an alternative therapeutic approach is to target the incretin pathway by extending the biological activity of incretin hormones with GLP-1 agonists (injectible peptides) or by preventing endogenous GLP-1 and GIP degradation with DPP-4 inhibitors (oral). The U.S. Food and Drug Administration (FDA) has thus far approved four incretin-based therapies for the treatment of type 2 diabetes.
Exenatide is a GLP-1 agonist (Byetta, Amylin/Eli Lilly, San Diego, Calif./ Indianapolis, Ind.). Sitagliptin (Januvia, Merck, Whitehouse Station, N.J.) and saxagliptin (Onglyza, Bristol-Myers Squibb/AstraZeneca, New York, N.Y./London, UK) are both DPP-4 inhibitors. Liraglutide (Victoza, Novo Nordisk, Bagsværd, Denmark) is a GLP-1 agonist. Vildagliptin (Galvus, Novartis, Basel, Switzerland), an orally active DPP-4 inhibitor, was approved in Europe in February, 2008.
A novel therapeutic known as MAR701 is being developed by Marcadia Biotech. This single peptide is an agonist of both GLP-1 and GIP. The great success of DPP-4 inhibitors suggests that activating both of these incretins hormones is a promising approach to treating type 2 diabetes.
Incretin-based therapies have shown success in improving glucose regulation without the undesirable side effects of hypoglycemia and weight gain observed with other diabetes drugs, including insulin. In particular, GLP-1 effects include enhanced insulin secretion, inhibition of glucagon secretion as well as inhibition of gastrointestinal secretion, motility, appetite, and food intake. Especially promising has been the potential for weight loss observed with GLP-1-based therapies. Obesity is one of the major risk factors for developing type 2 diabetes, and 85% of diabetes patients are overweight or obese. The goal of diabetes therapy should be to reduce blood glucose without causing weight gain or, even better, inducing weight loss.
Exenatide is a GLP-1 receptor agonist that requires inconvenient twice-a-day injections; an NDA for an extended-release formulation has been submitted to the FDA. The success of exenatide has been driven by not only efficacy in glucose control but also by mild weight loss, a fairly unique side effect for a diabetes drug. Liraglutide is a long-acting GLP-1 analog that is administered as an once-daily injection. Liraglutide also has demonstrated favorable effects on weight and systolic blood pressure. The most commonly reported adverse events and dose-limiting side effects for GLP-1 agonist drugs are nausea and mild gastrointestinal distress, observed in up to 40% of patients treated.
Several other GLP-1 receptor agonists are in late stage development. Taspoglutide (Roche, Basel, Switzerland) and Syncria (albiglutide, GlaxoSmithKline, London, UK) are both long-acting (weekly dosing) and in Phase 3 clinical trials.
DPP-4 inhibitors such as sitagliptin slow down the DPP-4-induced degradation of endogenously secreted GLP-1 and GIP, but the exposure to the incretins is limited by the endogenous secretion levels. Parenteral GLP-1 peptides allow much higher pharmacological levels than can be attained endogenously, but side effects of nausea and gastrointestinal distress are dose-limiting.
MAR701 targets both incretin receptors, GLP-1 and GIP, and may achieve a greater therapeutic index because GIP agonism is not associated with adverse gastric effects. In preclinical studies, MAR701 has been shown to lower blood glucose and induce weight loss without these dose-limiting side effects. MAR701 is currently in Phase 1 clinical studies.
Incretin-based drugs remain a promising approach to manage type 2 diabetes, and innovative pharmaceuticals and formulations of approved drugs are currently being studied in clinical trials. Additionally, the potential of GLP-1 targeting drugs for the treatment of obesity is under evaluation.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.