FDA's Upcoming Blockbuster Drug Decisions for Q4 2010
October 11, 2010
Again I am predicting the upcoming FDA Calendar for the next upcoming months for the Fourth Quarter. This is just an initial walkthrough of all the upcoming decisions. Lets see how well I do. I am doing pretty well from October's FDA calendar.
The FDA has some of its biggest decisions of the year looming in the next few months. The first new lupus drug in half a century, a blood-thinner with mega-blockbuster potential, controversial weight drugs and more are all up for regulatory decisions that can move markets and either throw a bucket of cold water or high octane gasoline on stock prices.
In every case, BioPharma Investor has been following the deals and data every step of the way--from early-stage development right through to the NDA and deal-making phases. Now everyone faces the moment of truth, or at least a complete response letter.
The FDA's final decision may be signaled by an expert panel vote, but this is one area where there really is no sure thing. The agency has appeared to be taking a tough stance on safety, which has bedeviled some late-stage therapies. And its complete response letters are always opened with a mix of dread and hope. We've seen plenty of surprising decisions so far this year, and there will likely be more in the months ahead.
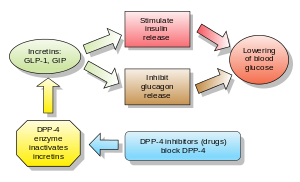
I have to do some more research on my Approval Decisions---Here is an preliminary list of FDA Approvals coming in the 4th Quarter of 2010. This is just an estimated guess off the top of my head. Some are toss-ups. The FDA is leaning more on the Complete Response Letter side which isn't necesarily a death sentence as why the drug was not approved and how it might be possible to get approval. Usually, it requires more Clinical Trials when a company receives a CRL from the FDA.
If you would like more Subscribe To BioPharma Investor for everyday stock news and up to date FDA decisions. Biopharma Investor
FDA Calendar Predictions
 Image via WikipediaGEN | News Highlights:Firms Report Promising Data for Type 1 and 2 Diabetes Candidates at 71st Annual ADA Meeting
Image via WikipediaGEN | News Highlights:Firms Report Promising Data for Type 1 and 2 Diabetes Candidates at 71st Annual ADA Meeting