FDA's Upcoming Blockbuster Drug Decisions for Q4 2010
October 11, 2010
Again I am predicting the upcoming FDA Calendar for the next upcoming months for the Fourth Quarter. This is just an initial walkthrough of all the upcoming decisions. Lets see how well I do. I am doing pretty well from October's FDA calendar.
The FDA has some of its biggest decisions of the year looming in the next few months. The first new lupus drug in half a century, a blood-thinner with mega-blockbuster potential, controversial weight drugs and more are all up for regulatory decisions that can move markets and either throw a bucket of cold water or high octane gasoline on stock prices.
In every case, BioPharma Investor has been following the deals and data every step of the way--from early-stage development right through to the NDA and deal-making phases. Now everyone faces the moment of truth, or at least a complete response letter.
The FDA's final decision may be signaled by an expert panel vote, but this is one area where there really is no sure thing. The agency has appeared to be taking a tough stance on safety, which has bedeviled some late-stage therapies. And its complete response letters are always opened with a mix of dread and hope. We've seen plenty of surprising decisions so far this year, and there will likely be more in the months ahead.
I have to do some more research on my Approval Decisions---Here is an preliminary list of FDA Approvals coming in the 4th Quarter of 2010. This is just an estimated guess off the top of my head. Some are toss-ups. The FDA is leaning more on the Complete Response Letter side which isn't necesarily a death sentence as why the drug was not approved and how it might be possible to get approval. Usually, it requires more Clinical Trials when a company receives a CRL from the FDA.
If you would like more Subscribe To BioPharma Investor for everyday stock news and up to date FDA decisions. Biopharma Investor
FDA Calendar Predictions
Stock investments ranging from Biotech, Pharmaceutical, and Medical Devices in the Healthcare Sector. Covering Clinical Trial recommendations and FDA Approvals.
Showing posts with label Amylin. Show all posts
Showing posts with label Amylin. Show all posts
10/14/10
Blockbuster FDA Decisions In The Next Few Months--FDA Calendar Predictions
Labels:
2010 Drug Approvals,
Amylin,
AstraZeneca,
AZN,
Biotech,
BMY,
Eli Lilly and Company,
FDA,
FDA Calendar,
HGSI,
Human Genome Sciences,
Pharmaceuticals,
Q4 Drug Approvals
10/12/10
Alkermes Gains FDA Approval For Vivitrol, Anticipating Oct 22 Approval with Amylin Pharmaceuticals
 Image via CrunchBase
Image via CrunchBaseAgain another winning prediction from my FDA calendar for October. Expect large gains for Alkermes tomorrow. I wouldn't sell the stock as they have another approval up on Oct. 22nd in conjunction with Amylin and Eli Lilly for Bydureon, a weekly injectible diabetes medication. I expect a FDA approval for that one too. But you never know with the FDA. Amylin already has approvals for daily injections but has modified the technology for weekly injections, so again I anticipate its approval as well. Good news for Alkermes.
Try Wikinvest here--http://www.wikinvest.com/
Wikinvest---Alkermes (ALKS)
Wikinvest--Amylin Pharmaceuticals (AMLN)
If you would like more Subscribe To BioPharma Investor for everyday stock news and up to date FDA decisions. Biopharma Investor
Alkermes addiction drug wins wider U.S. approval
Tue Oct 12, 2010 7:26pm EDT
* Vivitrol already used in U.S. for alcoholics
* Approval may help turn around money-losing drug
* Shares up nearly 69 percent in 2010
* Shares up another 4.2 percent after hours
Labels:
Addictions,
Alkermes,
Amylin,
Eli Lilly,
FDA approval,
Food and Drug Administration,
Opioid Dependence,
Substance abuse,
Substance dependence,
Vivitrol,
WikInvest
10/5/10
The Street's FDA Drug Approval Contest: October's FDA Calendar
The poll has 3 results:
1. Full Approval
2. Complete Response Letter (aka delayed approval or no approval)
3. No Decision (more time, the FDA has a lot of approvals in such a given short period)
Oct. 1: Hospira's(HSP) Dyloject for acute moderate to severe pain in adults. (CRL)---Big Company so no big deal, still a buy
Oct. 4: Human Genome Sciences'(HGSI) Zalbin for hepatitis C. (CRL--Lupus drug approval set for December so still a buy)
Oct. 11: Alexza Pharmaceuticals'(ALXA) AZ-004 for agitation in patients with schizophrenia or bipolar disorder. (I'm going with No Decision, short this stock, as this is their first FDA decision for the Staccato technology) Slightly leaning toward CRL but I will say No Decision as my final answer.
Oct. 11: Jazz Pharmaceuticals'(JAZZ) JZP-6 for fibromyalgia. (CRL--FDA panel killed this drug, it works but the FDA isn't approving a GHB type drug right now. Short this stock)
Oct. 12: Alkermes'(ALKS) Vivitrol for opioid addiction. (Full Approval--Jim Cramer would say Buy, Buy Buy)
Oct. 16: ISTA Pharmaceutical (ISTA) XiDay is simply aonce daily version of twice daily solution, and avoid generic competition (FDA Approval)
Oct 22: Arena Pharmaceuticals'(ARNA) lorcaserin for obesity. (No way in he##, CRL) I would recommend shorting this stock.
Oct 22: Amylin Pharmaceuticals(AMLN) (Partnered with Alkermes and Eli Lilly) Once weekly Diabetes Shot exenatide or Bydureon---This is a delay from CRL in March so I expect Full Approval this time around.
Oct 28: Vivus'(VVUS) Qnexa for obesity. (Give me a break, CRL) I don't care what data they have come up with, I would recommend Shorting this stock.
Oct 29: Avanir Pharmaceuticals'(AVNR) Zenvia for pseudobulbar affect. (I'm going to go with Full Approval although it's a toss-up)
Forest Labs(FRX) ceftaroline for Community Acquired Pneumonia. I can't find this on the FDA calendar---But I will say Full Approval even though I can't find it.
Oct 30: Biodel's(BIOD) Linjeta for diabetes. (No Decision, short this stock) Again on the fence, possible CRL.
Labels:
Alexza Pharmaceuticals,
Alkermes,
Amylin,
Arena,
Biodel,
FDA Calendar,
FRX,
Hospira,
Human Genome Sciences,
ISTA Pharmaceuticals,
Jazz Pharmaceuticals,
TheStreet,
Vivus
8/31/10
New Targets, Dual Agonists Increase Possibilities for Treating Diabetes
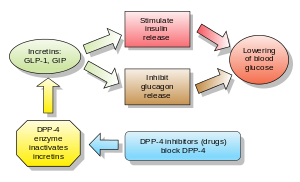 Image via Wikipedia Fritz French, Chief Executive Officer, Marcadia Biotech, Carmel, Ind.
Image via Wikipedia Fritz French, Chief Executive Officer, Marcadia Biotech, Carmel, Ind.Drug Discovery & Development - August 16, 2010
Diabetes is a common chronic disease affecting an estimated 285 million adults worldwide. This number will continue to grow as the population ages and becomes more obese—both risk factors for type 2 diabetes. The diabetes treatment market generated over $25 billion in 2009 and the number of patients is continuously rising.
Labels:
Amylin,
AstraZeneca,
Bristol-Myers,
Eli Lilly,
Galvus,
GSK,
Incretin Pathway,
Insulin,
Januvia,
Marcadia Biotech,
Merck,
Novartis,
Novo Nordisk,
Onglyza,
Roche,
Type 2 Diabetes,
Victoza
Subscribe to:
Posts (Atom)